The Cosmetic Regulatory Platform for Innovative Manufacturers & Growing Brands
Our Customers
Ithos is an extension of your R&D and regulatory team.
Create compliant products from start to finish faster, more accurate and easier than ever before.
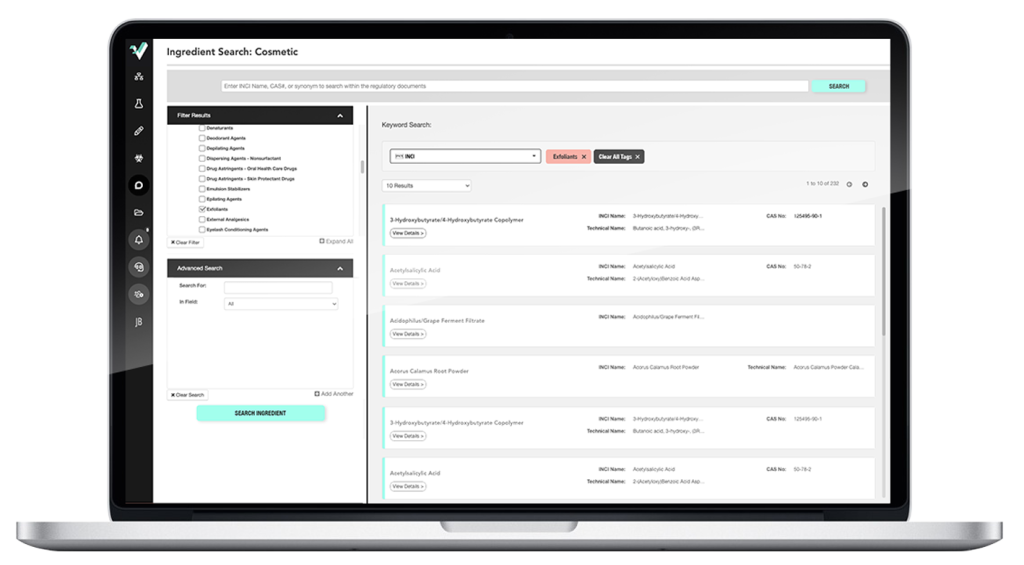
Integrate compliance checks into R&D to launch products faster.
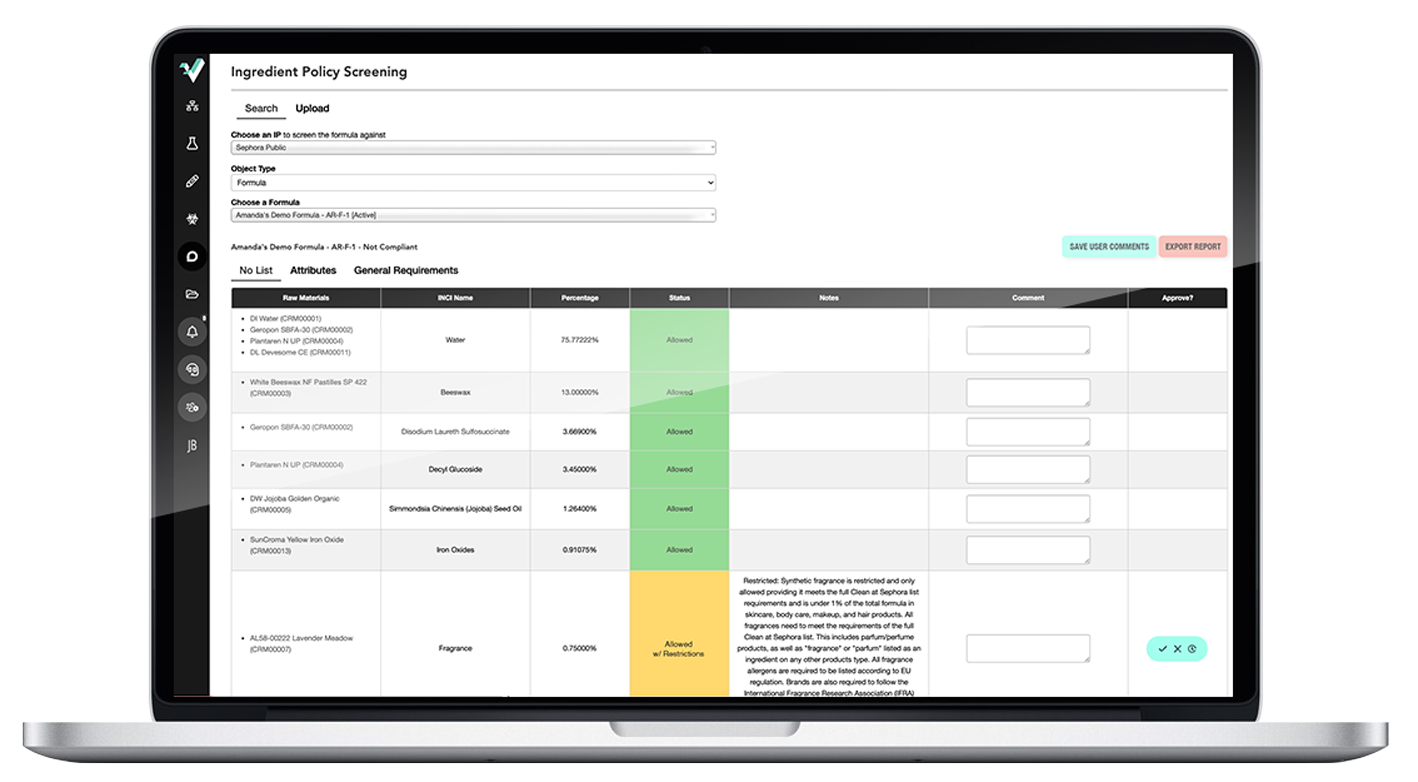
Ithos’s in-house regulatory team has over 75+ years of direct industry expertise.
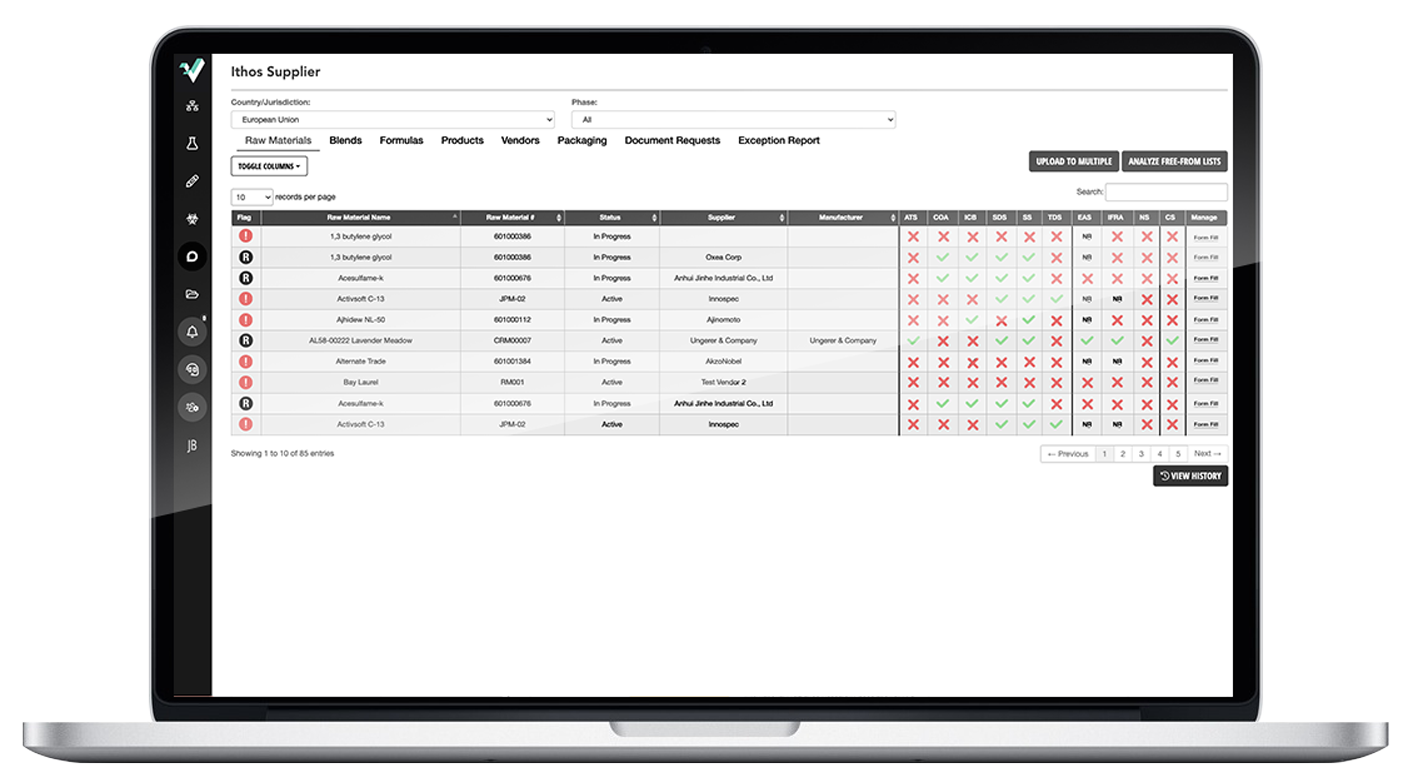
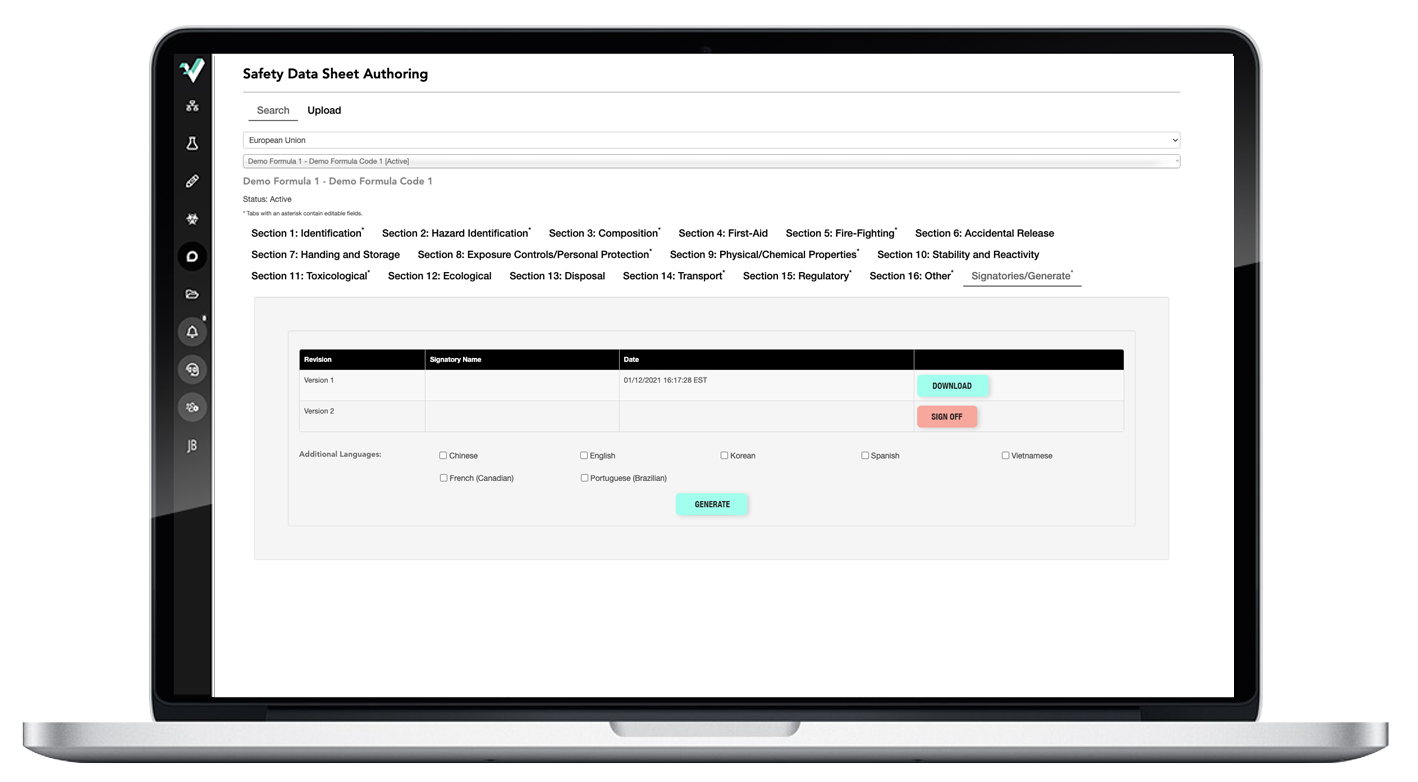
Easy-to-use SDS authoring tool creates WERCS-ready SDSs in minutes.
Join the hundreds of others that have taken their companies to the next level with Ithos.
Member Associations

Cosmetic Regulatory & Compliance Industry
Stay up-to-date on the latest regulatory news, webinars, and surveillance information for the Cosmetic Compliance Industry.
Enter your email below to receive our monthly newsletter directly to your inbox.









